Copper Evaporation During Low Pressure Carburizing
Introduction
Low pressure carburizing (LPC) in a vacuum furnace is increasingly the preferred method of
case hardening aerospace gears, with some alloys like Ferrium C61 and C64 have been designed
specifically for LPC. Several industry processes use acetylene as the principal carburizing gas.
Each LPC cycle has a series of carburizing boost steps, immediately followed by an equal
number of diffusion steps. A computerized controlled process using gas mass flow controllers
allows for rapid buildup of carbon at the surface of parts during the boost stage. The time for
each diffusion step increases throughout the cycle as a uniform case is formed, free of large
blocky and networked carbides. The length and number of each step is determined by the
material type, temperature, and case depth requirement.
Heating to the carburizing temperature with LPC can be performed in vacuum or using a partial
pressure gas. The pressures used in LPC processing typically ranges from 0.1 Torr (13.3 Pa) to
11 Torr (1.5kPa) (1). With some of the high alloy steels specified for aerospace parts, hydrogen
is often the preferred partial pressure gas to use during heat-up. Hydrogen reduces passive oxide
films at the surface, readily activating it for efficient and consistent carburizing (no preoxidation
treatment is required). In comparison to the boost stages that use patented or proprietary
acetylene/carrier gas mixtures for short periods of time, the total time for the diffusion stages is
relatively long. Like the heat-up stage, the diffusion stage can be done in vacuum or using a
partial pressure gas.
Selective case hardening is common with gears, where certain sections of a part are “stopped
off” or “masked” to prevent carburization at those locations. The intent is to have the masked
areas remain the same carbon weight percentage as the core base metal (figure 1). For aerospace
parts, the masking used is typically copper electroplating, which in instances covers much of the
surface area of a part. The low pressures and high temperatures used in LPC can lead to copper
evaporation during the diffusion steps. Copper vapor then condenses on colder components in
the hot zone, such as gas nozzles and ceramic insulators. In a worst-case scenario, copper
deposits have caused arcing and melting of power feed-throughs damaging the furnace and in
some instances the parts (figure 2).


The use of partial pressure gas helps suppress evaporation is well-established in the heat treating
industry (2,3). This present study investigates the effect of temperature, pressure, and carrier gas
species on the amount of copper evaporation that occurs from copper foil test samples.
Experimental Details
Oxygen-free high conductivity copper foil, 0.002” thick, was cut into 2 inch square coupons.
Three coupons were prepared for each test process. Just prior to placing the coupons into the
heat treat furnace, they were briefly immersed in a solution of Almco 2510TM (citric acid
solution) to remove surface oxidation and contaminants. They were rinsed in distilled water,
methanol, and dried with compressed nitrogen gas. Each sample was weighed using an
analytical balance that records up to four decimal points in grams. Upon removing coupons from
the heat treat furnace they were immediately reweighed using the same scale. The measured
weight loss indicates the capability of the gas/partial pressure combination to suppress copper
evaporation.
A graphite insulated vacuum furnace with a cylindrical, vertical hot zone, 10″ diameter x 18″
high, was used for this study. The three coupons were hung on separate stainless steel wires
attached to the lid of the furnace. All cycles began after an initial pump down to 1 x 10-4 Torr
(1.3 x 10-2 Pa) to assure vacuum integrity. Three temperatures were chosen for testing: 1575°F
(857°C), 1700°F (927°C), and 1825°F (996°C), to cover the common LPC temperature range.
Four atmosphere types were used for processing: vacuum at <1 x 10-3 Torr (<13.3 x 10-2 Pa),
partial pressure nitrogen, argon, and hydrogen, at 0.25 Torr (33.3 Pa), 2.5 Torr (333.3 Pa), and
10 Torr (1.3 kPa). The partial pressure was set prior to heating and maintained during the 6-hour
hold time at temperature. All samples were cooled in nitrogen at 625 Torr (83.3kPA)
A temperature uniformity survey (TUS) was performed in a horizontal graphite insulated LPC
production furnace with a hot zone 24” (0.6 m) x 24” (0.6 m) x 72” (1.8 m). Following the
AMS2750E protocol, a nine point TUS was performed for each gas and pressure to compare
with the baseline data at pressures less than 1 x 10-3 Torr (<13.3 x 10-2 Pa).
Results:
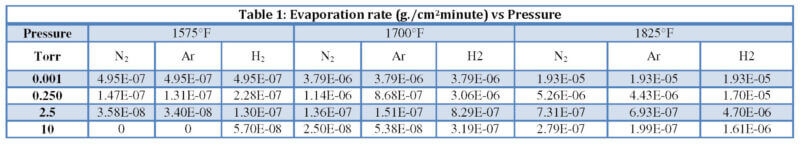
The average rate of copper evaporation for each temperature, gas species, and pressure is listed
in Table 1. The evaporation rates versus pressure curves in Figure 3 are representative of all
three temperatures studied. Copper evaporation drops off sharply in nitrogen and argon, even at
pressures as low as 0.25 Torr (33.3 Pa). Argon is slightly more suppressive than nitrogen; however, hydrogen is considerably less effective at reducing metal vapor losses at the
temperatures studied. The data reveals that copper evaporation decreases with a decrease in
temperature and an increase in pressure. Figure 3 also shows a flattening of the curve as pressure
increases indicating that suppression slows down with continued increase in pressure.
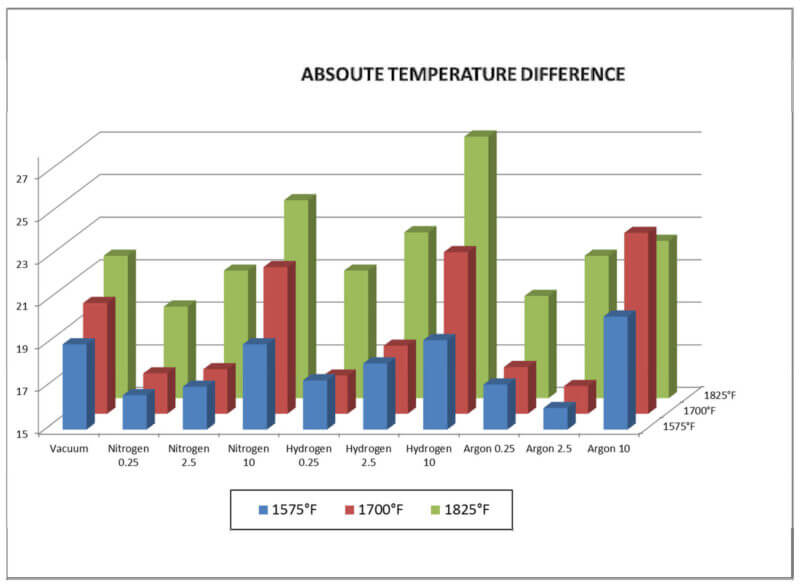
Temperature data reveals that the uniformity of the furnace degrades as temperature increases
(figure 4). Compared to high vacuum, <1 x 10-3 Torr (13.3 x 10-2 Pa), temperature uniformity
improved with all gases at 0.250 Torr (33.3 Pa) or 2.5 Torr (333.3 Pa). However, at 10 Torr (1.3
kPa), for all gases, uniformity was inferior to high vacuum. Generally, the work thermocouple
temperatures decreased as the pressure increased in the furnace. This is not unexpected as the
gas flow rates and convective losses are greater as the pressure increases.
Conclusions:
The stringent temperature uniformity requirements of the aerospace industry play an important
role in selecting the partial pressure gas to minimize evaporation. Nitrogen at 2.5 Torr is the
most economic and effective gas for minimizing copper evaporation. Argon, was marginally
more effective at suppressing evaporation compared to nitrogen, however, nitrogen enhanced the
temperature uniformity compared to argon. The use of nitrogen gas is increasingly important to
use during longer time diffusion steps as the LPC temperature increases to minimize copper
evaporation and copper contamination of furnace components.
Authors: Trevor Jones, Principal Engineer; Virginia Osterman, Ph.D., Senior Scientist; Donald Jordan, FASM, Corporate Metallurgist – Solar Atmospheres
1. Heuer, Volker, Low Pressure Carburizing; ASM Handbook Volume 4A, ASM
International, Materials Park, Ohio; 2013
2. Herring, Daniel, Using Partial Pressure in Vacuum, Industrial Heating; November 2005
3. Jones, William, Partial Pressure Vacuum Processing – Part I and II, Industrial Heating,
September and October 1997