What is Vacuum?
When we talk in terms of heat treating in vacuum, most people think we do so in a space entirely devoid of matter. In reality, this isn’t true. In practical terms then, a vacuum is a space with a highly reduced gas density. Just how many gas molecules are still present and how they react inside the vacuum furnace is something we should better understand. Let’s learn more.
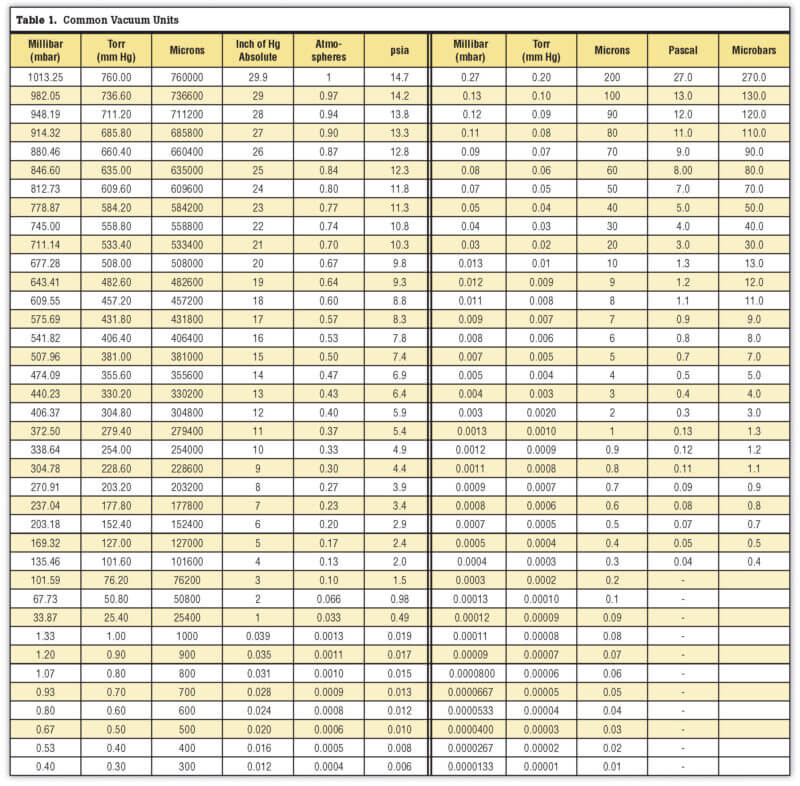
 Common Vacuum Units
Common Vacuum Units
Pressure in vacuum applications is commonly measured in microns or Torr here in the U.S. or millibar (mbar) elsewhere. A Torr is 1/760th of atmospheric pressure. Atmospheric pressure, or the pressure exerted by the weight of air on a column of mercury, at standard temperature (0ºC) and pressure (sea level) is 29.9 inches or 760 Torr (1013 mbar).
One of the mystifying things about vacuum and vacuum furnaces, especially in the heat-treating industry in the U.S., is the confusing way in which vacuum units are used. Devices installed on furnaces often measure in different units, which force us to think in terms of microns, Torr, inches of mercury, millibar, bar, microbar, Pascal, kilopascals, inches of water column and atmospheres (psia or psig)! This is extremely confusing, especially to those who are not familiar with vacuum terminology. To avoid confusion, try to stay with one unit of measure, converting everything to this common base. To help, the conversions between common vacuum units are shown in Table 1.
A Little Vacuum Theory
Most of us know that a gas is a collection of molecules and that most gases are mixtures of many different kinds of molecules. Gas molecules are in constant motion. The higher the temperature, the faster these molecules move, and as one might expect, the motion of gas molecules dramatically slows down as a gas is cooled. With an increase in temperature, there is an increase in kinetic energy (or energy of motion). Molecular collisions occur between molecules. If enclosed, these molecular collisions also occur against the walls of their container, resulting in a pressure rise. In other words, pressure is simply the force per unit area that a gas exerts on the walls of its container.
Avogadro (1811) determined that a mole of any gas occupies a volume of 22.4 liters and contains 6.02 x 1023 molecules at standard temperature and pressure. To create a vacuum in any closed vessel, therefore, some of the gas molecules must be removed.
Now for a surprise and an important concept. At atmospheric pressure, one cubic centimeter of air contains approximately 2.69 x 1019 molecules all moving around in a random motion. As you might expect, this results in a fantastic number of collisions. In other words, the mean free path between molecules (or the average distance a molecule can travel before colliding with another molecule) is only about 2.6 x 10-6 inches. So if we pump a one-cubic-centimeter volume down to a micron (1 x 10-3 Torr), which is a vacuum level commonly used in heat treating, we still have about 3.54 x 1013 molecules, or well over half of them remaining! You might be wondering how this can be an acceptable condition for heat treating, especially when about 20% of those remaining molecules are oxygen? The answer is that the mean free path increases dramatically, reducing the probability of molecular collision with the surface of the workpiece.
If we continue to pump down to say a thousandth of a micron (1 x 10-6 Torr), the level of a good diffusion-pumped vacuum furnace, the number of molecules per cubic centimeter is still 3.54 x 1010, but the mean free path increases to over 30 miles (48 km). The path of the molecules is ultimately limited by the walls of the vessel and not by the collisions between molecules. Flow as we know it doesn’t exist. This is the reason for the relatively large opening and piping used for diffusion pumping systems. If the opening to the diffusion pump weren’t that large, any molecules that migrated into the pump suction stream would rebound and move to other parts of the chamber. By comparison, at 200 miles above the earth, the vacuum is 1 x 10-8 Torr. At 400 miles it is 1 x 10-10 Torr, and in deep (or outer) space it is 1 x 10-16 Torr.
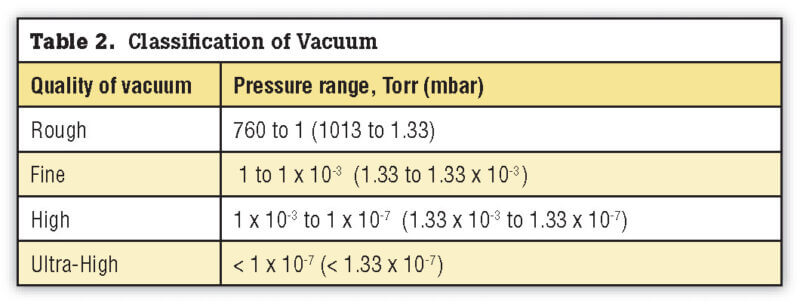
What is a Good Vacuum?
Simply stated, the more molecules that are removed, the better the vacuum. The quality of a vacuum is described by the degree of reduction in gas density, i.e., gas pressure. One distinguishes four different vacuum levels or qualities as shown in Table 2. The heat treatment of steel is carried out in three of these – namely rough, fine and high. The majority of applications are processed in the fine vacuum range.
Author: Daniel Herring
Sources:
1. Brunner Jr., William F. and Batzer, Thomas H., Practical Vacuum
Techniques, Robert E. Krieger Publishing Company, 1974
2. Kimball, William H., Vacuum…is it really nothing? C. I. Hayes
Inc., 1977.
3. Steel Heat Treatment Handbook 2nd Edition, edited by Totten,
George E. and Howes, Maurice .A. H., Chapter 7: Vacuum Heat
Processing, Marcel-Dekker, 1997.

 Common Vacuum Units
Common Vacuum Units